In Class 12 Boards there will be Case studies and Passage Based Questions will be asked, So practice these types of questions. Study Rate is always there to help you. Free PDF Download of CBSE Class 12 Chemistry Chapter 13 Amines Case Study and Passage Based Questions with Answers were Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Case Study Questions Amines to know their preparation level.
Amines Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 12 Chemistry Chapter 13 Amines
Case Study/Passage-Based Questions
Case Study 1:RCONH2 is converted into RNH2 by means of Hoffmann bromamide degradation. During the reaction, the amide is treated with Br2 and alkali to get amine. This reaction is used to descend the series in which carbon atom is removed as carbonate ion (CO32–). Hoffmann bromide degradation reaction can be written as :
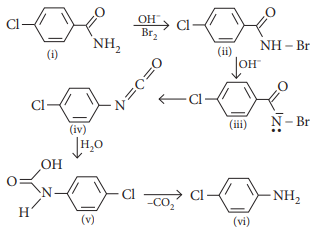
Hoffmann bromamide degradation is used for the preparation of
(a) primary amines (b) secondary amines
(c) tertiary amines
(d) secondary aromatic amines
Answer:(a) primary amines
Which is the rate-determining step in Hoffmann bromamide degradation?
(a) Formation of (i) (b) Formation of (ii)
(c) Formation of (iii) (d) Formation of (iv)
Answer:(d) Formation of (iv)
Which of the following is used for the conversion of (i) to (ii)?
(a) KBr (b) KBr + CH3ONa
(c) KBr + KOH (d) Br2 + KOH
Answer:(d) Br2 + KOH
What are the constituent amines formed when the mixture of (i) and (ii) undergoes Hoffmann bromamide degradation?
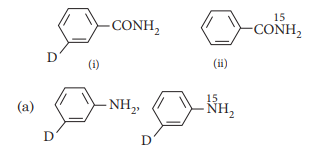

Answer:(b)
Case Study/Passage-Based Questions
Case Study 2:The amines are basic in nature due to the presence of a lone pair of electrons on the N-atom of the –NH2 group, which can donate to electron-deficient compounds. Aliphatic amines are stronger bases than NH3 because of the +I effect of the alkyl groups. The greater the number of alkyl groups attached to the N-atom, the higher the electron density on it and more will be the basicity. Thus, the order of the basic nature of amines is expected to be 3° > 2° > 1°, however, the observed order is 2° > 1°3°. This is explained on the basis of crowding on the N-atom of the amine by alkyl groups which hinders the approach and bonding by a proton, consequently, the electron pair which is present on N is unavailable for donation and hence 3° amines are the weakest bases. Aromatic amines are weaker bases than ammonia and aliphatic amines. Electron-donating groups such as –CH3, –OCH3, etc. increase the basicity while electron-withdrawing substitutes such as – NO2, –CN, halogens, etc. decrease the basicity of amines. The effect of these substituents is more at p than at m-positions.
Which one of the following is the strongest base in an aqueous solution?
(a) Methyl amine
(b) Trimethyl amine
(c) Aniline
(d) Dimethyl amine
Answer:(d) Dimethyl amine
Which of the following order of basicity is correct?
- (a) Aniline > m-toluidine > o-toluidine
- (b) Aniline > o-toluidine > m-toluidine
- (c) o-Toluidine > aniline > m-toluidine
- (d) o-Toluidine < aniline < m-toluidine
Answer:(d) o-Toluidine < aniline < m-toluidine
What is the decreasing order of basicity of primary, secondary, and tertiary ethylamines and NH3?

Answer:(d)
The order of basic strength among the following amines in benzene solution is
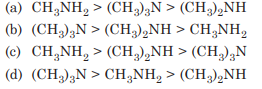
Answer:(b)
Case Study 3: When the mixture contains the three amine salts (1°,2° and 3°) along with quaternary salt, it is distilled with KOH solution. The three amines distill, leaving the quaternary salt unchanged in the solution. Then the mixture of amines is separated by fractional distillation, Hinsbergs method, and Hoffmann’s method.
(i) Hinsberg reagent is
| (a) aliphatic sulphonyl chloride | (b) phthalamide |
| (c) aromatic sulphonyl chloride | (d) anhydrous ZnCl2 + conc. HCl. |
Answer:(c) aromatic sulphonyl chloride
(ii) Primary amine with Hinsberg’s reagent forms
| (a) N-alkyl benzene sulphonamide soluble in KOH solution |
| (b) N-alkyl benzene sulphonamide insoluble in KOH solution |
| (c) N,N-dialkyl benzene sulphonamide soluble in KOH solution |
| (d) N,N-dialkyl benzene sulphonamide insoluble in KOH solution. |
Answer:(a) N-alkyl benzene sulphonamide soluble in KOH solution
(iii) To separate amines in a mixture Hoffmann’s method is used. The Hoffmann’s reagent is
| (a) benzenesulphonyl chloride | (b) diethyloxalate |
| (c) benzeneisocyanide | (d) p-toulenesulphonic acid. |
Answer:(b) diethyloxalate
(iv) 30 amines with Hinsberg’s reagent give
| (a) no reaction | (b) product which is same as that of 10 amine |
| (c) product which is same as that of 2° amine | (d) products which is a quaternary salt. |
Answer:(a) no reaction
Hope the information shed above regarding Case Study and Passage Based Questions for Class 12 Chemistry Chapter 13 Amines with Answers Pdf free download has been useful to an extent. If you have any other queries about the CBSE Class 12 Chemistry Amines Case Study and Passage-Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible.
By Team Study Rate

