In Class 11 Final Exams there will be Case studies and Passage Based Questions will be asked, So practice these types of questions. Study Rate is always there to help you. Free PDF Downloads of CBSE Class 11 Physics Chapter 11 Case Study and Passage-Based Questions with Answers were Prepared Based on the Latest Exam Pattern. Students can solve Class 11 Physics Case Study Questions Thermal Properties of Matter to know their preparation level.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.
In CBSE Class 11 Physics Paper, There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Thermal Properties of Matter Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 11 Physics Chapter 11 Thermal Properties of Matter
Case Study/Passage-Based Questions
Case Study 1:
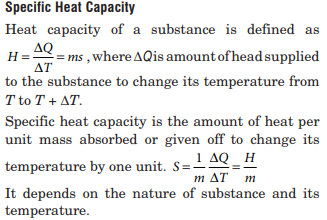
Which one of the following substances has the highest specific heat capacity at room temperature and atmospheric pressure?
(a) Water
(b) Ice
(c) Aluminium
(d) Mercury
Answer: (a)Water
The heat capacity of a substance is infinite. It means
(a) heat is given out.
(b) heat is taken in.
(c) no change in temperature whether heat is taken in or given out.
(d) all of these.
Answer: (c) no change in temperature whether heat is taken in or given out.
Water is used as a coolant because
(a) it has a lower density.
(b) it has low specific heat.
(c) it has high specific heat.
(d) it is easily available.
Answer: (c) it has high specific heat.
Calorie is defined as the amount of heat required to raise the temperature of 1 g of water by 1°C and it is defined under which of the following conditions?
(a) From 14.5°C to 15.5°C at 760 mm of Hg
(b) From 98.5°C to 99.5°C at 760 mm of Hg
(c) From 13.5°C to 14.5°C at 76 mm of Hg
(d) From 3.5°C to 4.5°C at 76 mm of Hg
Answer: (a) From 14.5°C to 15.5°C at 760 mm of Hg
Find the thermal capacity of 40 g of aluminum. (s = 0.2 cal/g K)
(a) 168 J/°C (b) 672 J/°C
(c) 840 J/°C (d) 33.6 J/°C
Answer: (d) 33.6 J/°C
Case Study 2: States of matter viz: solid, liquid, and gas are functions of temperature and heat content. During the change of state of a substance, the exchange of heat takes place between the substance and its surrounding. In this process temperature of the substance remains constant. At a certain temperature known as the melting point. Both the solid and liquid states of the substance coexist in thermal equilibrium. Similarly, at boiling point both the liquid and vapor states of the substance co-exist in the thermal equilibrium. There are certain substances which on heating directly pass from solid to vapor state without passing through the liquid state. This is a sublimation process in which the solid changes to the vapor state of the substance. The process of change of state depends on pressure and temperature.
The states of matter, such as solid, liquid, and gas, are dependent on what factors?
a) Density and Mass
b) Temperature and Heat Content
c) Volume and Weight
d) Pressure and Density
Answer: b) Temperature and Heat Content
What happens to the temperature of a substance during its state change?
a) It increases continuously
b) It decreases continuously
c) It fluctuates randomly
d) It remains constant
Answer: d) It remains constant
At the melting point, which states of a substance coexist in thermal equilibrium?
a) Solid and Liquid
b) Liquid and Gas
c) Solid and Gas
d) All states
Answer: a) Solid and Liquid
At the boiling point, which states of a substance coexist in thermal equilibrium?
a) Solid and Liquid
b) Liquid and Gas
c) Solid and Gas
d) All states
Answer: b) Liquid and Gas
What is the process called when a substance changes directly from a solid to a gas without passing through the liquid state?
a) Condensation
b) Evaporation
c) Sublimation
d) Vaporization
Answer: c) Sublimation
What factors does the process of change of state depend upon?
a) Density and volume
b) Pressure and temperature
c) Mass and weight
d) Heat content and melting point
Answer: b) Pressure and temperature
Hope the information shed above regarding Case Study and Passage Based Questions for Class 11 Physics Chapter 11 Thermal Properties of Matter with Answers Pdf free download has been useful to an extent. If you have any other queries about the CBSE Class 11 Physics Thermal Properties of Matter Case Study and Passage-Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible.
By Team Study Rate



